We met with 4 different water treatment companies this week.
In Santa Barbara, most of our water comes from the Santa Ynez Valley watershed, which feeds Cachuma Lake. There is almost no farming or industrial activity in the water shed, so our water is reasonably pure. However, it tends to go through a lot of rock which means it picks up quite a lot of minerals, and is relatively hard; we’ve been told it is about 23 grains hard. It also tastes pretty bad. Additionally, a new reverse osmosis treatment plant just came online which will feed about a third of the city water system.
The main things we are concerned about are
- hard water, which is very hard on shower surfaces and fixtures
- the bad taste
- chlorine and other remnants of the treatment process (chloramines)
Here is what we are thinking for a treatment system.
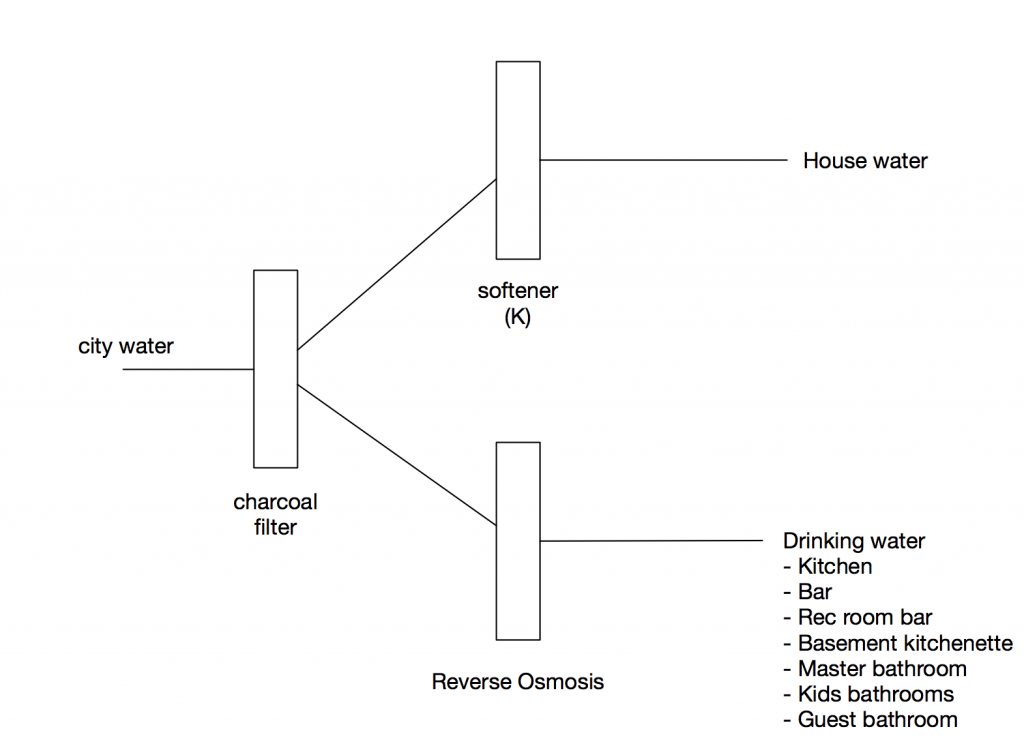
The charcoal filter removes the chlorine and other byproducts
The water softener removes the minerals which is better for the pipes, fixtures, and shower surfaces. This week I finally understood how water softeners work, and it is interesting.
A water softener, also called an ion exchange system, has two main parts
- A bed of resin beads
- A salt tank / reservoir for recharging the system
The salt brine in the recharge tank is flushed through the resin beads. The sodium ions cling to the resin beads. When water flows through the resin bed, the calcium and other minerals are exchanged on the resin beads for the sodium ions. So, the resin beads capture the minerals and free the sodium. Every once in a while, the recharge brine is flushed through the resin bed which reverses this process; the minerals are removed from the resin beads and replaced again with sodium ions, and the resulting mineral (and sodium) rich brine is flushed down the sewer.
There are two main implications from this process
- You are putting sodium into your water. It isn’t a good idea to drink sodium rich water or water plants with it.
- Flushing a high sodium brine down the sewer is very hard on the municipal water system. It makes it harder to treat and any resulting recovered grey water is very salty, making it bad for plants. Many cities are banning water softeners.
Some will tell you that there isn’t much sodium in the water, and it is fine to drink it and water plants, but it all depends on your water hardness. The harder your water, the more sodium you will use and therefore add to your water.
Another salt that works almost as well as sodium chloride is potassium chloride. Unlike sodium, potassium is actually good for you and beneficial for plants. However, potassium salt is about 4 times as expensive right now, and it doesn’t work quite as well as sodium salt. It also isn’t bad for the municipal water system.
Despite the higher cost, we are going to use potassium as it is the right thing to do.
The reverse osmosis unit will filter for drinking water, mostly to improve the flavor. One drawback of reverse osmosis is that, by removing all the minerals from the water (which increase the PH), it makes the water more acidic. Acidic water will eat away at copper pipes. There are two ways around this problem.
- Use plastic pipes
- Re-mineralize the water
We are still deciding whether to use plastic pipes or not. From what we know, plastic pipes (PEX) carry a slightly higher risk of failure.
Re-mineralization is the process of adding a very small amount of minerals back to the water, mostly calcium, which makes the water more alkaline.
Another consideration is the trend of alkaline water. By removing the hardness from our drinking water, we are making it less alkaline.
We are still deciding on all this. We may end up plumbing the house to accommodate the system above, but not putting it in initially.
Good analysis…. Some people don’t like the taste of RO water and mitigate that by adding minerals back into the flow stream for taste, which also raises the ph.